24+ Calculating Ph Of A Buffer
So pKa is equal to 925. The pH scale is used to measure the acidity or basicity of a solution.

Buffer Ph Calculations And Buffer Solutions Medium
The pH of a buffer is determined by two factors.

. List the values you are given. Now I believe you can refer to the solution given in your book. This answer is the same one we got using the acid dissociation.
Steps for Calculating the pH of a Buffer Step 1. An alkaline buffer solution has a pH greater than 7. Calculating the pH of a buffer 4129 views Apr 16 2021 How to use the Henderson-Hasselbalc Show more 23.
A pH buffer solution is an aqueous solution capable of maintaining its pH stable against the addition of small quantities of strong acids and bases. A buffer or buffered solution is one that resists a change in its pH when H or OH ions are added or removed owing to some other reaction taking place in the same. If you compare it with a 11 buffer where the pH pKa it decreased because there is a higher concentration of acid.
Is going to give us a pKa value of 925 when we round. Calculating the pH of a buffer - YouTube 000 235 ALEKS. Calculating the pH of a buffer solution requires the Henderson-Hasselbalch equation and knowledge of the concentrations in molarity of both the weak acid and its conjugate base.
PH pKa log A- HA The Henderson-Hasselbalch equation enables determination of a buffer solutions pH when the pKa is known. CA Concentration of. Calculate the concentration of the respective constituents in the equilibrium and then proceed with plugging.
Calculate the pH of a buffer solution. 1 The equilibrium constant Ka of the weak acid and 2 the ratio of weak base A - to weak acid HA in solution. PH -log 42 x 10 -7 log 003500035 pH 638 1 738 Therefore the pH of the buffer solution is 738.
Acids and Bases pH of a Buffer pH of a Buffer Henderson Equation Calculator K a Acid Dissociation Constant. So the pH of our buffer solution. 1 Different weak acids have.
This behavior is of. It ranges from 0 to 14 with 7 being neutral and values above 7 indicating alkaline solutions and values below. Example of calculating the pH of solution that is 100 M acetic acid and 100 M sodium acetate using ICE table.
942K views 8 years ago. What will be the pH of a buffer solution that contains 0240 M propanoic acid pKa 488 p K a 488 and 0300 M lithium propanoate. Another example of calculating pH of a soluti Show.
CB Concentration of the Conjugate Base. So were gonna plug that into our Henderson-Hasselbalch equation right here. Alkaline buffer solutions are commonly made from a weak base and one of its salts.
1 A buffer solution. Use the values given in the Henderson-Hasselbalch equation to solve for pH. Decrease or increase depends on your frame of reference.
7 24 Calculating Ph Of Buffer Solutions Henderson Hasselbalch Equation Chemistry Libretexts
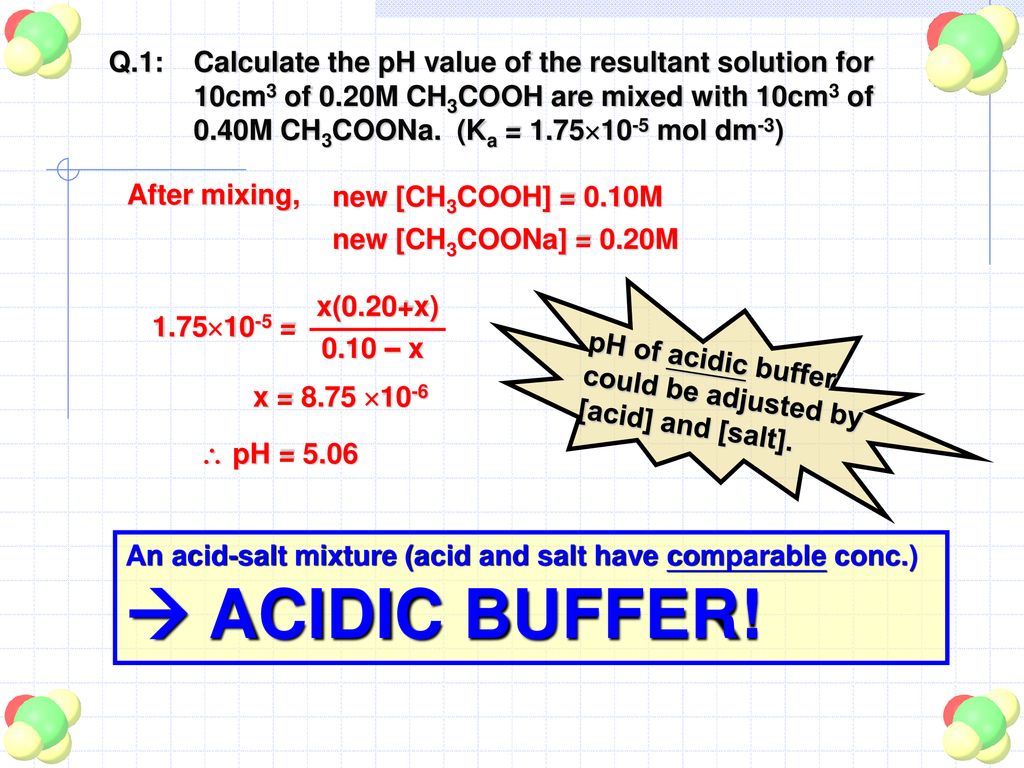
Buffer Solutions What Is A Buffer Solution Definition Ppt Download

Calculate Ph Of Buffer Solution

A Level Calculating The Ph Of A Buffer Solution Calculations Of Amounts Needed Gce As A2 Chemistry Revision Notes Ks5 Pdf Buffer Solution Acid Dissociation Constant

Calculating The Ph Of Buffer Solutions Youtube

How To Calculate The Ph Of A Buffer Solution Youtube
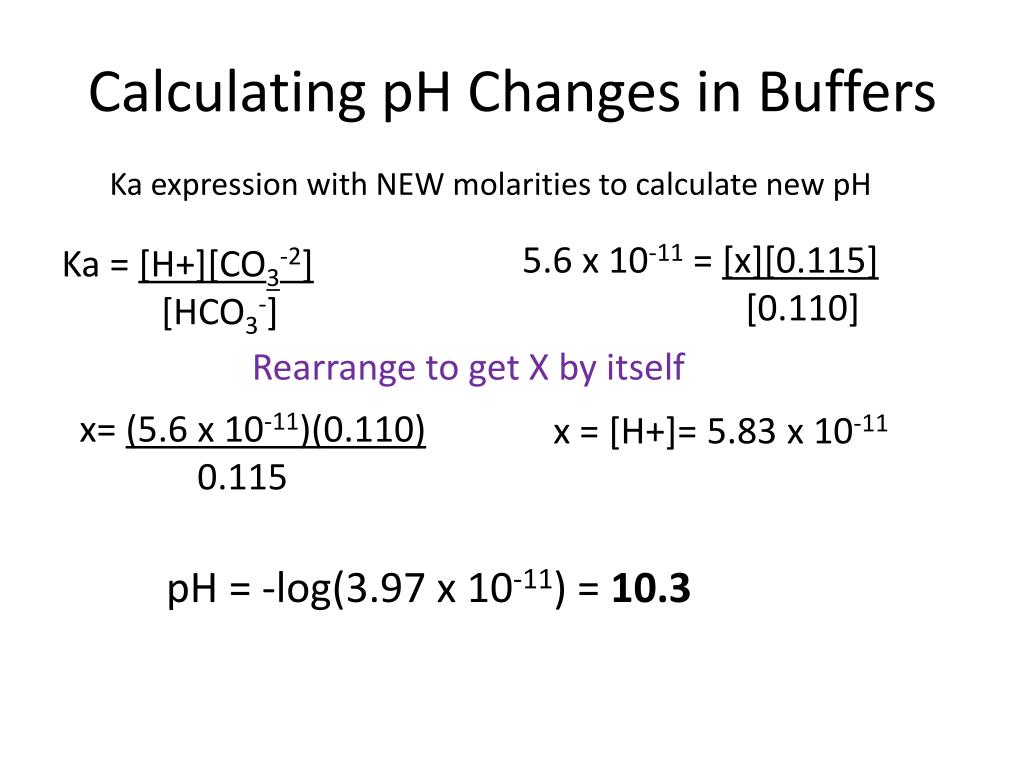
Ppt Entry Task Feb 12 Th Tuesday Powerpoint Presentation Free Download Id 1994617

How To Calculate The Ph Of A Buffer Solution Fully Worked Example Youtube

Chapter 16 Aqueous Ionic Equilibrium Ppt Download

1 How Much Does The Ph Of A Buffer Change When An Acid Or Base Is Added Though Buffers Do Resist Change In Ph When Acid Or Base Are Added To
![]()
Step By Step Approach To Arterial Blood Gas Analysis

Buffer Ph Calculations And Buffer Solutions Medium
![]()
Step By Step Approach To Arterial Blood Gas Analysis

1500 Problems In Chemistry Pdf Titration Chemistry
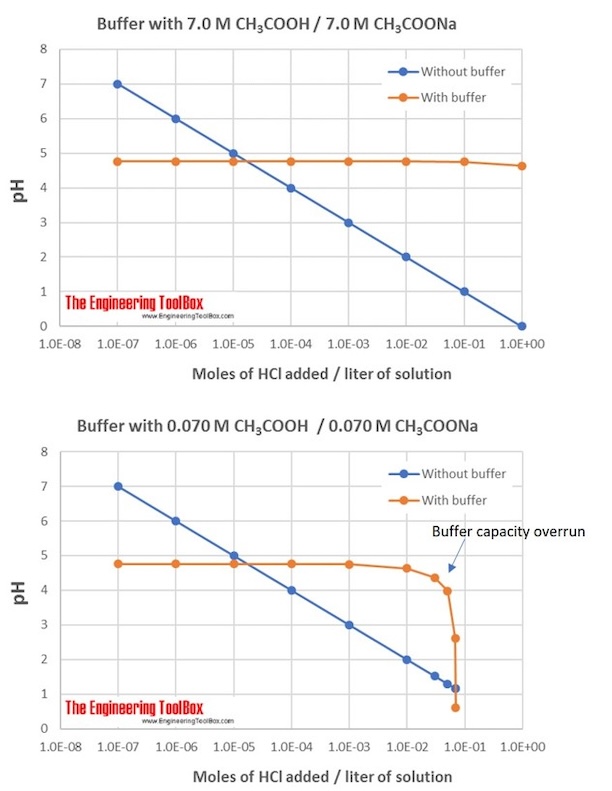
Buffer Solutions

1 How Much Does The Ph Of A Buffer Change When An Acid Or Base Is Added Though Buffers Do Resist Change In Ph When Acid Or Base Are Added To

11 9 Buffers A Buffer Solution Maintains The Ph By Neutralizing Small Amounts Of Added Acid Or Base An Acid Must Be Present To React With Any Oh Added Ppt Download